The Solid-State Battery Working Principle
Solid-State Battery Working
The solid-state battery (SSB) is a type of rechargeable battery that stores electricity and releases it during usage. The SSBs work by storing energy in the form of ions and can improve energy density and power density. Various SSEs have been developed over the last few decades and can be grouped into three main types. These are inorganic, organic and composite electrolytes. Inorganic electrolytes usually have high thermal stability and ionic conductivity, while inorganic ones tend to be low in thermal stability.
The solid-state battery working principle consists of a cathode composite layer, a sulfide solid electrolyte layer, and an energy-dense anode. The energy dense anode consists of discrete micro-scale Silicon particles. Lithium ions move from the anode to the cathode as positive charges are deposited. The positive Lithium ions move from the aneroid to the cathode via the sulfide solid electrolytae. The sulfide electrode is made of a stable two-dimensional interface between the Lithium-Si alloy particle and the Li-Si electrode.
Manganese Market Analysis by Martin Kepman CEO of Manganese X Energy Corp
The solid-state battery working principle is not entirely understood yet. However, the concept is fairly simple and has many potential applications. A typical example of a lithium battery is a ball that is being rolled up a hill. The ball requires energy to roll up the hill. The more energy the ball has to move up the hill, the more energy it can store. As the ball rolls downhill, the lithium ions diffuse into carbon particles and release energy.
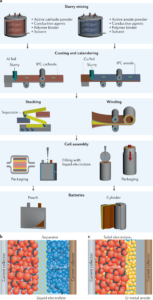
The Solid-State Battery Working Principle
A solid-state battery has four main problems. The first is the slow growth of lithium dendrites, which form when the anode is placed near the anode. The second is the slow process of interface formation. As a result, the contact between the particles is lost during electrochemical cycling. These three problems are overcome by the new battery technology. You can now use your mobile phone or tablet without a problem.
The solid-state battery works by transferring electricity from an anode to anode. When a battery is used, electrons must pass through an electrolyte. This is the process by which a lithium-ion cell stores energy. The negative electrode is connected to anodes and receives electrons from the anode. The anode is connected to the positive electrode. The negative electrode is connected to the anode. The battery can store electricity when a charging cycle is complete.
The solid-state battery is a very effective form of energy storage. It uses electrodes made of lithium metal and uses electrolytes that are made from solid-state materials. These batteries can be very durable, which means that the lithium metal will last for years. The new system can be recharged many times, which will make it more convenient for users. They can also be portable, which is an added benefit. So, you can use them anytime you want.